
😎 Understanding chemical Introduction to (video
Multicomponent systems, chemical potential 15 Chemical equilibrium 16 Temperature, pressure and K p 17 Equilibrium: application to drug design 18 Phase equilibria — one component. Introduction to reaction kinetics 31 Complex reactions and mechanisms 32 Steady-state and equilibrium approximations 33 Chain reactions 34.
PPT Chemical PowerPoint Presentation, free download ID624912
CHEMICAL KINETICS PPT. - Google Slides CHEMICAL KINETICS DAY 1 CHEMICAL KINETICS DAY 2 Factors that Affect the Reaction Rate Constant Temperature: At higher temperatures, reactant.
PPT Chemical PowerPoint Presentation, free download ID5408395
Chemical kinetics- Physical Chemistry | PPT Chemical kinetics- Physical Chemistry Jul 15, 2021 • 1 like • 3,385 views S Sanchit Dhankhar Education The branch of chemistry, which deals with the study of reaction rates and their mechanisms, called chemical kinetics.

PPT Chemical Lecture notes edited by John Reif from PPT
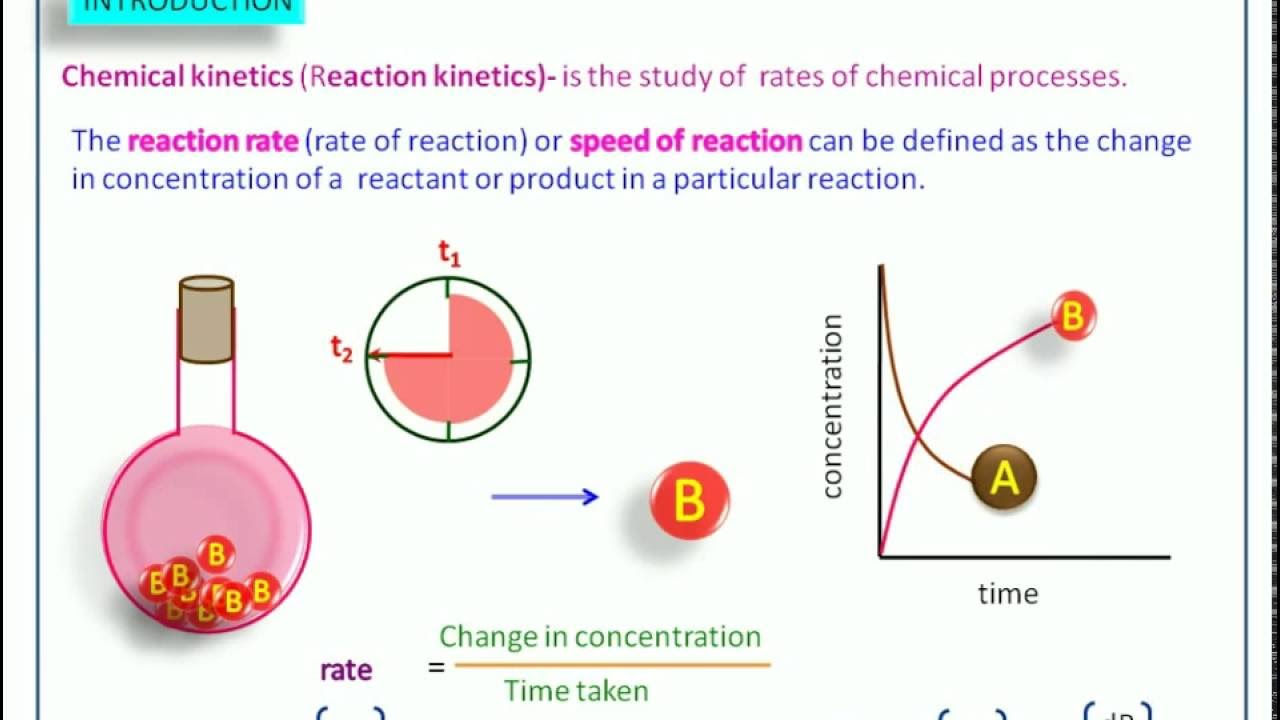
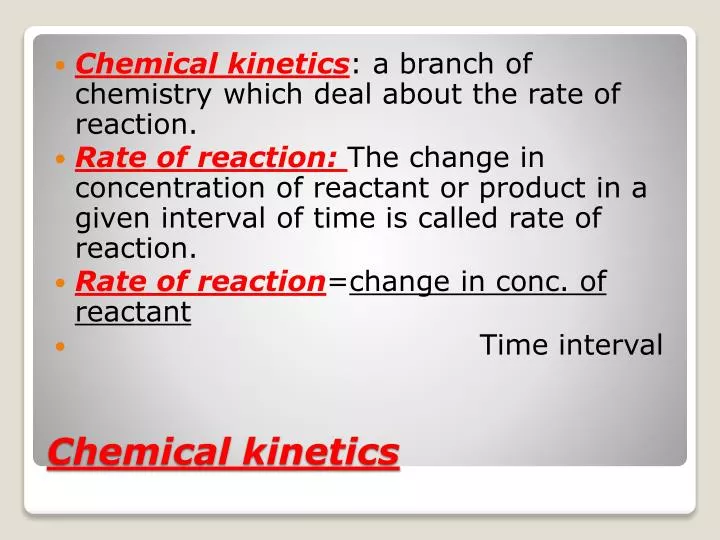
2. What is chemical kinetics. 3. Reaction Rates Rate of a chemical reaction = change in concentration (mol/L) of a reactant or product with time (s, min, hr); Rate of Reaction=. 4. Chemical Kinetics A B ∆ [A] rate = ∆t ∆ [A] = change in concentration of A over time period ∆t ∆ [B] rate = ∆t ∆ [B] = change in concentration of B.

PPT Chemical PowerPoint Presentation, free download ID2054453
Mar 31, 2019. 650 likes | 768 Views. Chemical Kinetics. Unit 11. Factors affecting reactions Collision Model Activation energy, activated complex Exothermic/endothermic reactions Energy of reactions ∆E Reaction Rate Average reaction rate Instantaneous reaction rate Kinetics Stoichiometry Reaction Rate and Concentration. Download Presentation.
PPT Chemical PowerPoint Presentation, free download ID5408395
Chapter 14 Lecture- Chemical Kinetics. Jan 15, 2010 • 156 likes • 24,808 views. Education Technology. Chapter 14 Lecture on chemical kinetics. M. Mary Beth Smith Teacher at El Molino High School.

PPT Chemical PowerPoint Presentation, free download ID2720862
Chemical Kinetics The study of reaction rates; How fast does a reaction proceeds and what factors affecting it; A measure of the change of the concentration of a reactant (or a product) as a function of time. The study of rate yields information on the mechanism by which a reaction occurs at molecular level. Types of Rates Initial Rates Rates.
Chemical Introduction (with Animation) YouTube
Chemical kinetics biotech energy pvt limited. 92.5K views • 39 slides. Reaction Kinetics Yujung Dong. 5.4K views • 65 slides. Chemical kinetics Gandaki Boarding School,Lamachaur-16 Pokhara, Nepal. 10.1K views • 34 slides. Theories of Reaction Rate - Ashika G Bebeto G. 10.9K views • 15 slides.
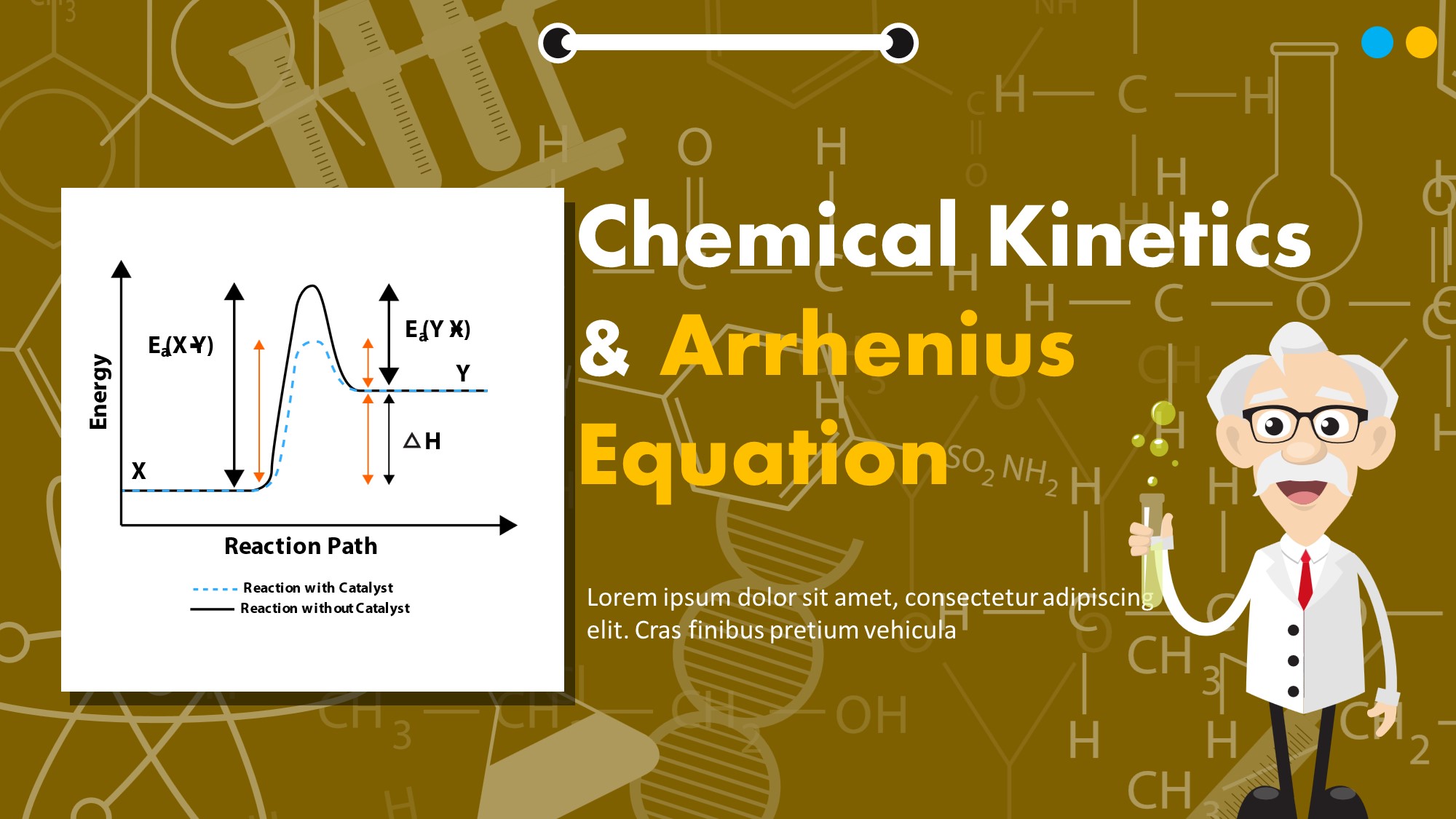
Free Chemical Presentation MyFreeSlides
Learn the basic concepts and principles of chemical kinetics from this PowerPoint presentation by Duke University. It covers topics such as reaction rates, rate laws, reaction mechanisms, catalysis, and more. This is a useful resource for students who are studying ch 14 chemical kinetics.
PPT Chemical PowerPoint Presentation, free download ID5409963
P05 Chemical Kinetics Edward Blurock. 5.3K views • 50 slides. CBSE Class 12 Chemistry Chapter 4 (Chemical Kinetics) | Homi Institue - Download as a PDF or view online for free.

PPT Chemical PowerPoint Presentation, free download ID4983216
To print or download this file, click the link below: Chapter 12 - Chemical Kinetics.ppt — application/vnd.ms-powerpoint, 9.10 MB (9539584 bytes)

Chemical Chapter 14 YouTube
PPT - Chemical Kinetics PowerPoint Presentation, free download - ID:1157066 Download Presentation Download 1 / 34 Download Presentation >> Chemical Kinetics Feb 25, 2013 420 likes | 590 Views Chemical Kinetics. Chung (Peter) Chieh Professor of chemistry University of Waterloo Waterloo, Ontario, Canada. A B. rate = . D [A]. D [B]. rate = -. D t.
PPT Chem.414 Physical Chemistry II PowerPoint Presentation, free
Chemical Kinetics Outline: Kinetics Reaction Rates How we measure rates. Rate Laws How the rate depends on amounts of reactants. Integrated Rate Laws How to calc amount left or time to reach a given amount. Half-life How long it takes to react 50% of reactants. Arrhenius Equation How rate constant changes with T. Mechanisms Link between rate and molecular scale processes.
PPT Chemical PowerPoint Presentation, free download ID4273230
Chapter 13Chemical Kinetics • Factors that affect reaction rates • How to express reaction rates • Rate Laws • Effects of temperature reaction rates • Mechanisms of reactions • Catalysis. Kinetics • Studies the rate at which a chemical process occurs. • Besides information about the speed at which reactions occur, kinetics also sheds light on the reaction mechanism (exactly how.
PPT Chemical PowerPoint Presentation, free download ID1907290
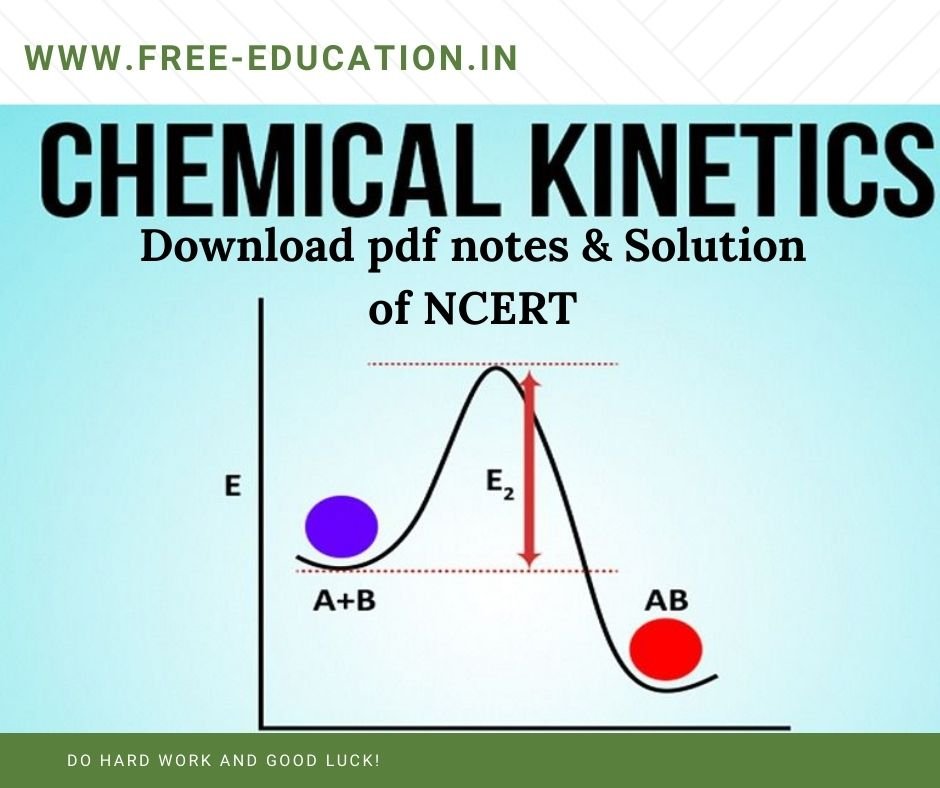
Q2. The value of rate constant for the decomposition of ethyl iodide is 1.6 x 10-5 s-1 at 327 °C and 6.36 x 10-3 s-1 at 427 °C. Calculate the activation energy for the reaction. (R = 8.314 JK-1 mol-1) Q3. The rate of a particular reaction quadruples when the temperature changes from 293K to 313K.
chemical pdf notes Archives Wisdom TechSavvy Academy
PPT - Chemical Kinetics PowerPoint Presentation, free download - ID:5408395 Presentation 1 / 72 Download Presentation >> Chemical Kinetics Oct 11, 2014 1.48k likes | 3.21k Views Chemical Kinetics. By- A.P.S. BHADOURIYA M.Sc. ( Lko . Uni. ) , B.Ed. NET (UGC-CSIR). Chemical Kinetics.